Stainless steel is a key material in the energy industry, and its properties make it ideal for applications such as hydrogen transport. As govern-ments around the world set ambitious net-zero goals, the use of hydrogen as a clean fuel is becoming increasingly important to reduce carbon emissions in the transportation sector. In fact, many countries have included hydrogen as a central element in their climate change strategies for transportation. For instance, the European Union aims to have 40GW of renewable hydrogen electrolyzers by 2030, and hydrogen is expected to play a major role in achieving its decarbonization goals. Governments are investing heavily in the development of hydrogen technologies for transportation, and significant progress has been made in recent years. Research groups like the Hydrogen Council and H2ME have conducted extensive trials to investigate the feasibility and benefits of using hydrogen as a fuel for transportation, and their findings have been encouraging. These developments present an opportunity for the stainless steel industry to play a key role in the energy transition by providing the materials needed for hydrogen transport infrastructure.
By Tuncay Kurtulan, Senior Materials Engineer, OGC Energy
Preventing Hydrogen-Induced Damage
Natural gas distribution networks typically operate at relatively low pressures and temperatures, which are not expected to cause significant material degradation in the presence of hydrogen. Non-metallic and metallic parts have been evaluated for their performance within a temperature range of 0 to 65°C and a maximum pressure of 100 bar, which represents the common operating conditions under investigation.
Although guidelines like API RP 941 offer valuable recommendations for the safe utilization of materials in high-pressure and high-temperature hydrogen environments, it is important to note that these guidelines specifically ad dress the risk of high-temperature hydrogen attack (HTHA) and other damage mechanisms that typically occur at temperatures above approximately 200°C. These guidelines are particularly applicable to refinery settings where such risks are present.
While the effects of hydrogen on the degradation of soft and other materials in natural gas distribution networks are generally considered negligible, it is crucial to assess the compatibility of materials for hydrogen transport infrastructure to ensure safe and reliable operations.
The term ‘hydrogen embrittlement’ is commonly used to describe different damage mechanisms that occur when metals absorb hydrogen.
The recognized mechanisms of hydro-gen-induced damage such as hydrogen stress cracking (HSC) can occur in susceptible materials after hydrogen absorption. Sulfide stress cracking (SSC) is another form of HSC where H2S expo-sure is required for hydrogen adsorption. Galvanically induced hydrogen stress cracking (GHSC) and hydrogen-induced cracking (HIC) are typically only susceptible to rolled products. The shape and amount of MnS inclusions are the decisive factors for HIC. Stress-oriented hydrogen-induced cracking (SOHIC) is the same damage mechanism as HIC, however, the HIC cracks are aligned according to stress. Lastly, high-temperature hydrogen attack (HTHA) occurs at temperatures above 204°C.
It is crucial to consider these mechanisms in the design and selection of materials for hydrogen transport infrastructure to prevent hydrogen-induced damage and ensure safe and reliable operations.


Understanding the Risk of Hydrogen Embrittlement
Special attention should be given to rolled products and materials containing MnS inclusions, which are susceptible to HIC and SOHIC. In addition, GHSC is a potential risk for materials that are in contact with dissimilar metals or alloys. By taking these factors into account and selecting appropriate materials and designs, the risk of hydrogen-induced damage can be minimized, and hydrogen can be safely and effectively transported.
This statement implies that hydrogen stress cracking (HSC) is a credible failure mechanism for hydrogen gas, and there may be a few uncommon cases where hydrogen-induced cracking (HIC) occurs.
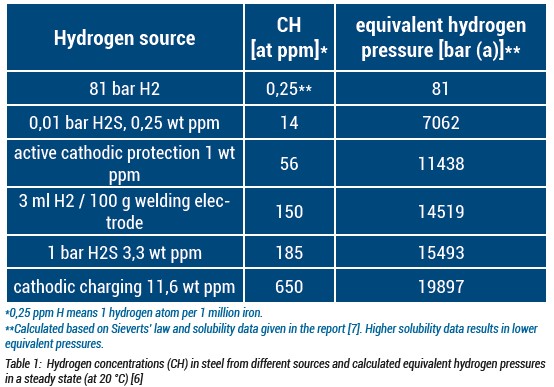
The risk of hydrogen embrittlement can be associated with the expected hydrogen concentration. With that in mind, the expected hydrogen concentration in the steel that is subject to 81 bar H2 is calculated and compared with other steels that are subject to different hydrogen charging environments within the table reproduced from CEN/TR 17797.
Based on the information presented in Table 1, the hydrogen pressures corresponding to the measured concentrations were calculated. Assuming a maximum pressure of 81 bar in natural gas pipelines, the estimated hydrogen concentration in steel was found to be 0.25 ppm, which suggests that the impact of hydrogen gas at this pressure can be deemed insignificant. However, it is important to note that metals highly susceptible to hydrogen stress cracking (HSC) may still undergo cracking even when exposed to trace amounts of hydrogen.
The NACE MR 0175 / ISO 15156 standards offer comprehensive guidance to restrict hydrogen-enhanced dam-age mechanisms by specifying material limits for sour service. However, these standards do not include specific mandates for partial pressures below 0.05psia (0.3 kPa). Even though hydro-gen transportation equipment and piping are not required to conform to sour service standards, certain concerns remain pertinent for any environment.
There is a high risk of cracking for materials that are highly susceptible to sulfide stress cracking (SSC) and hydrogen stress cracking (HSC), such as high nickel steels and martensitic alloys. High-strength steels with yield strength above 140ksi (>965N/mm2) can also suffer from HSC. Additionally, designs with stress concentrations increase the risk of cracking.
Studies have shown that the fatigue strength of certain steels may be reduced even in low-pressure hydrogen environments. While hydrogen absorption may not necessarily lead to hydro-gen-enhanced cracks, it can still cause changes in the mechanical properties of the metal being studied.
According to Sandia’s Technical Reference for Hydrogen Compatibility of Materials, the presence of hydrogen gas can lower fatigue resistance, even at low pressure, which can be harmful to machinery exposed to cyclic loads. For an example, the large bore gas transportation valves are infrequently actuated. Therefore, fatigue-related failures are unlikely to occur in.


Commonly Used Stainless Steel in The Gas Grid
Since transported hydrogen and natural gas are dry and do not possess corrosive properties, alloys such as duplex and super duplex are not preferred to combat internal corrosion for gas transportation purposes. However, components that are sensitive to dimensional tolerances or subject to a corrosive external environment may require the use of corrosion-resistant alloys such as stainless steel.
Stainless steel is a commonly used material in various industries, including construction materials for valves. Type 304 and 316 are low-strength materials that are generally not prone to hydrogen embrittlement, even in high-severity environments like sour service.
There may be rare instances of susceptibility to hydrogen embrittlement in some cases, such as centrifugally cast or wrought material in solution-annealed seal rings and gaskets. As the operating temperatures of the gas network are not within the range susceptible to high-temperature hydrogen attack (HTHA), grades such as 347 are not typically utilized.
Martensitic grades, like 17-4 PH, can be utilized where better mechanical properties are necessary. Compared to austenitic or martensitic stainless steel, 17-4 PH is relatively cheap, has high strength, and excellent corrosion resistance. As a result, it is widely used in the petrochemical, aviation, and nuclear industries. However, experience and research have shown that 17-4 PH is highly sensitive to hydrogen. Significant increases in crack growth rates in fatigue have been reported at pressures as low as 2 atm hydrogen gas. This trend can be seen in other martensitic grades, such as 410 and 440C, with reduced ductility and notched tensile strength test results at 69 MPa or even below 1 atm hydrogen gas.

Conclusion
Although hydrogen can affect the mechanical properties of metals, even at low pressures, it does not necessarily pose harm to all exposed metals. In the case of hydrogen transportation through gas distribution networks, it is not expected to have detrimental effects. Hence, there is currently no requirement to employ advanced materials specifically for this purpose.
The current standards have limited specifications for material limits regarding hydrogen gas. However, it is expected that the upcoming API 6Z standard will address this issue for valves, although the extent of its coverage is currently unknown. Association for Materials Protection and Performance (AMPP) recently initiated new standards projects under SC26 to address hydrogen related topics. In light of this, manufacturers should be aware of the expected stresses for their products subject to hydrogen and assess the associated risks accordingly until adequate guidance is established by a reputable standard organization.
By adopting a proactive approach and implementing appropriate measures to prevent hydrogen-induced damage, the safe and reliable transport of hydrogen can be ensured. The transition to a more sustainable energy system is achievable by minimizing the risks of failure or catastrophic incidents.


