The market for air, water, and energy products used in blood plasma fractionation, and the development of drugs derived from the plasma, is approaching $5 billion per year. Alongside this, the market for AWE stainless products is approaching $80 million dollars per year.1
By Bob Mcilvaine, The Mcilvaine Company
Stainless steel products are critical to minimizing the total cost of operations. Purchasers need to know what products have the Lowest Total Cost of Ownership (LTCO) in each niche. Blood plasma fractionation is composed of many unique niches which must be analyzed.
Compared to many pharmaceutical processes, plasma fractionation is a large volume application. Nearly 100 million liters of plasma are produced each year, sourced from donors globally.
Due to its complicated processes, fractionation technology is very complex. As a result, plasma donated in developing countries is often discarded and only the red blood is utilized. Man-made recombinant products have been making inroads into the market, though the human-derived plasma product market is still growing at approximately 6% per year.
CSL and Grifols generate over 40% of the industry revenues. Takeda, Octapharma, and Kedrion are also in the top ten suppliers. The air, water, energy stainless product opportunities at each company have to take into consideration other products and also contract manufacturing. Most of the $8 billion in revenues generated by CSL are tied to fractionation products. On the other hand, Takeda, with revenues of $32 billion is a larger stainless purchaser, even though their fractionation related sales are less than $3 billion.
The technology necessary for fractionation is currently undergoing significant changes. Even the cold ethanol process itself is being challenged by newer technologies. The need to analyze all the technology options in this industry is therefore heightened.
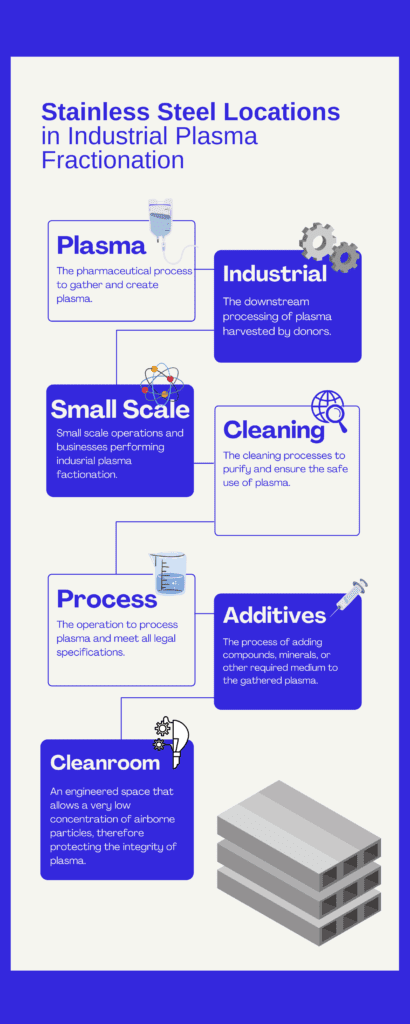
Processes Involved in Blood Fractionation
Fractionation requires that donated blood first be separated into its component parts, namely platelets and plasma. The processes for industrial plasma fractionation processes differ from those used in small scale operations.
Stainless steels are used for clear liquids, slurries and gas/liquid combinations. Stainless steel is also used in creating the ultrapure water (water for injection) needed for the Clean-in place or other cleaning systems. There are dilution fluids and various additives used in the fractionation process, however stainless steel is used in conjunction with storage, filtration, and purification processes.
Distillation and membrane filtration are two routes for achieving acceptable water purity. Both processes use stainless steel, however more stainless steel is used with distillation technology.
How Stainless Steels are Used
Mainly four types of stainless steels are used in pharmaceutical equipment manufacturing; 304, 304L, 316 and 316L. Stainless steel 316 and 316L are used to construct the equipment in direct contact with the pharmaceutical products including water system tanks and pipelines, while 304 is used for the non-contact parts.
Single-use can be justified if downtime is avoided or efficiency is improved. However, this is only true if the product price is high, so this would not apply to plasma, but rather would apply to some the plasma products. For example, in developing countries where plasma may otherwise be discarded, single-use could be justified, though single-use is just one more option where total cost of ownership needs to be determined. Festo and Biotest considered both nickel plated and stainless fittings for the 6,000 valves in a new Biotest fractionation facility.
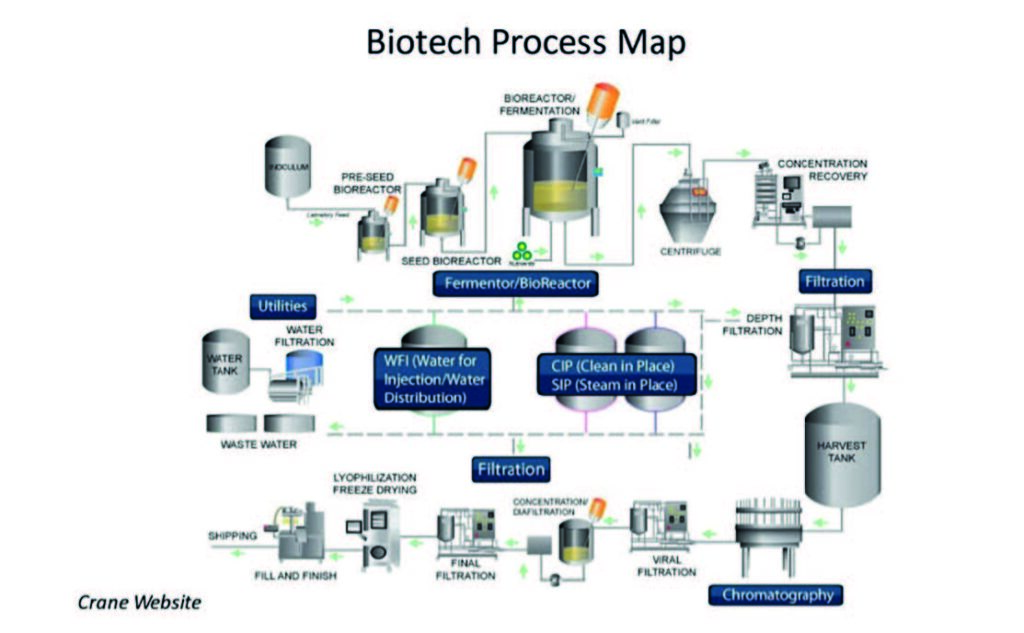
There is also the issue of scale. Companies that manufacture very large volumes of a drug may have to use stainless steel because there are limits to the capacities offered by single use. For instance, for a single-use bioreactor, 2000 L is the most practical size. For many companies this is enough, but some manufacturers need to have larger systems. Larger single-use bio-reactors do exist, but handling a large bag is more challenging and the consequences, should a leak occur, have been considered too great. Stainless steel tanks definitely do not pose a leak risk like single-use. A number of tanks, filters, separators, pumps, and valves are made from stainless steel as shown in the process map.
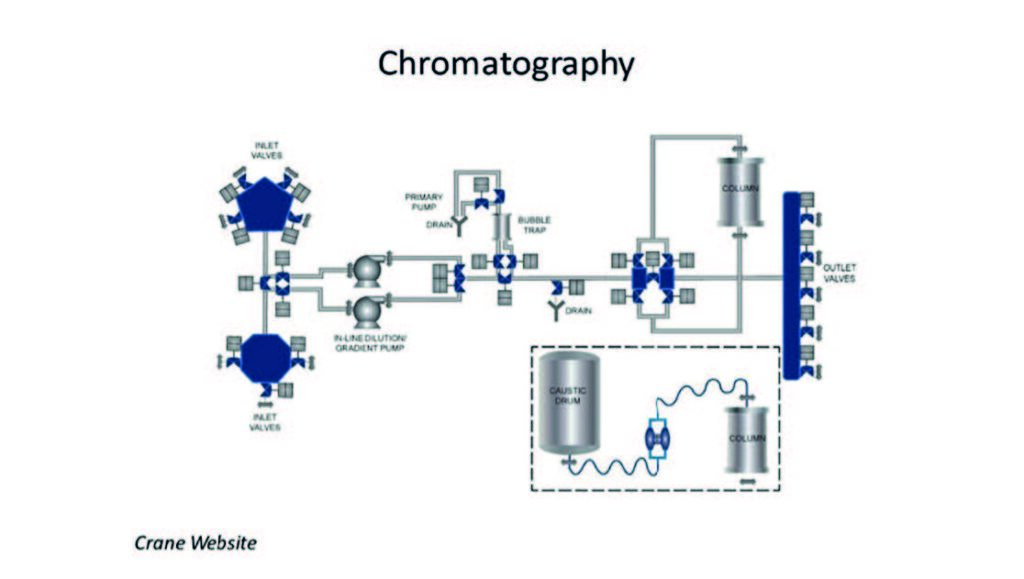
The SGE annealed type 304 chromatography stainless steel is a high-quality material that is specially produced and cleaned for chromatography use. It has a very smooth, non-contaminated bore. Longer lengths are supplied in 6” diameter coils and enclosed in a stainless steel spiral mount with ferrules. Ideal for producing stainless steel capillary columns and for HPLC connecting tubing.
Single-use tubing, connectors, and valve components are among the components that can eliminate cleaning-in-place and sterilizing-in-place (CIP/SIP) procedures, removing these costly and time-consuming steps that contribute to downtime and, thereby, increasing manufacturing throughput and productivity of the facility. Time and personnel once used for CIP/SIP of stainless steel process lines can now be reallocated to production, increasing the overall efficiency of the manufacturing process.
Stainless Steel Product Examples
GEA
With the hycon®, GEA has developed a stainless concept that allows the use of a self-cleaning, fully automatic separator, combined with a consistent separation of mechanical components, i.e. motor and drive, and the area in contact with the product. In this completely enclosed three-room concept, the suspended bowl with hood and solids trap is located in a clean room mounted below the separator frame, while the drive units are located in a lowerclassified room above. The operating elements are also located outside and thus emission-free through the separation from the clean room. The very valuable solids are discharged fully automatically from the suspended separator bowl at reduced or full operating speed under sterile conditions.
Strassburger
Filter presses have stainless frames and components. Both the multilayer filter SF and the SF CLEANSYSTEM by Strassburger Filter Press are designed to meet the unique demands of the pharmaceutical industry. Stainless steel is used specifically to meet the high demands for hygiene and purity.
SPX Flow
SPX Flow makes stainless steel pumps for fractionation processes. With advanced features of the Universal 2 PD pump, the Universal 2 BioPharmaceutical is designed for the special requirements of pharmaceutical and biotech processes. Features include 316L stainless steel rotors standard; non-galling Waukesha “88” alloy rotors optional 316L stainless steel product contact surfaces standard; 316L stainless steel or electropolish of all product contact surfaces optional Stainless steel bearing frame.
Any sanitary agitator, and blending vessel should be specified and designed to allow proper cleaning and sterilizing per GMP and BPE standards. Mixing tanks are generally fabricated in USP VI PP (polypropylene) or FDA PE (Polyethylene) materials, or stainless steel 316Lss grade, with polished surfaces <20ra and post fabrication passivation and electropolishing. All materials must be traceable and have proper gmp documentation part of the mixer turnover package.
Conclusion
There are many niches, suppliers, and products utilizing stainless steel in the fractionation processes. However, non-stainless single use alternatives are carving out significant market share. Single use has cost advantages for small quantities, but large scale production will continue to rely on stainless steel. Some innovations are needed to keep stainless steel competitive. For example, an extra stainless centrifuge bowl can be supplied. Even though the additional capital cost can be high, there is no downtime for bowl cleaning. This eliminates one of the disadvantages of permanent systems. Therefore, costs need to be analyzed in order to achieve the lowest total cost of ownership in the manufacturing of fractionation products. Due to the changing technology and market, the need for analysis is continuous. Suppliers have the opportunity to respond to the opportunities to reduce costs and to develop and sell products with higher EBITA. In this manner, both the purchaser and supplier benefit.
References
1. Stainless Steel Markets for AWE Products published by the Mcilvaine Company
About the Author
Robert McIlvaine is the CEO of the McIlvaine Company, which publishes Industrial Valves: World Markets. He was a pollution control company executive prior to 1974 when he founded the present company. McIlvaine oversees a staff of 30 people in the U.S. and China. http://www.mcilvainecompany.com



